Search This Blog
A Dental Blog With The Latest Dental News & Dental Technology For Your Dental Practice.
Posts
Showing posts from February, 2008
Denture Cleanser Allergic Reactions and Misuse
- Get link
- X
- Other Apps
Lava™ Scan ST Design System to work with other technologies and/or systems
- Get link
- X
- Other Apps
3M ESPE Introduces Improved Speed and Efficiency in Automatic Mixing
- Get link
- X
- Other Apps
Lead Contamination in Off Shore Dental Laboratory Restorations
- Get link
- X
- Other Apps
What was cool at the Chicago Mid-Winter Meeting
- Get link
- X
- Other Apps
Laser light may be able to detect diseases on the breath
- Get link
- X
- Other Apps
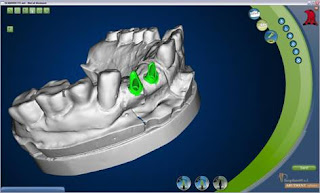
3M ESPE Debuts Lava™ Chairside Oral Scanner C.O.S.
- Get link
- X
- Other Apps
Organization for Safety and Asepsis Procedures (OSAP) announces their 2008 Symposium
- Get link
- X
- Other Apps
Recovering photos from a corrupted flash card
- Get link
- X
- Other Apps
Accuracy of magnetic resonance imaging compared with computed tomography for implant planning
- Get link
- X
- Other Apps
Zenith Dental to Unveil Complete Post and Core Restorative System
- Get link
- X
- Other Apps
Probe of Insurance Companies For Setting Low UCR's
- Get link
- X
- Other Apps
1st International Computer Controlled Local Anesthesia Delivery Summit
- Get link
- X
- Other Apps
Cannabis Smoking and Periodontal Disease Among Young Adults
- Get link
- X
- Other Apps
THE DANGERS OF ORAL DISEASE IN CHILDREN
- Get link
- X
- Other Apps
ZENITH DENTAL TO INTRODUCE LUXACORE® Z-DUAL
- Get link
- X
- Other Apps
When and How Often You Consume Acidic Foods or Beverages Affects Dental Health
- Get link
- X
- Other Apps
BioCAD To launched at the Mid-Winter in Chicago
- Get link
- X
- Other Apps