Short implants compared to regular dental implants after bone augmentation in the atrophic posterior mandible: umbrella review and meta-analysis of success outcomes
International Journal of Implant Dentistry volume 9, Article number: 18 (2023)
Abstract
Purpose
To assess the body of evidence of short versus regular implants after bone augmentation (BA) in the atrophic posterior mandible in the context of implant treatment success outcomes.
Methods
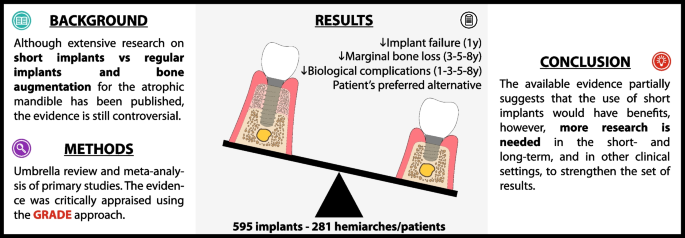
Seven databases, two registries, and reference lists were searched for systematic reviews and meta-analysis (SR/MA), randomized controlled trials (RCTs) and longitudinal studies published in English, Spanish or German since 2012. Confidence in the SR/MA methodology was evaluated using AMSTAR-2 and the risk of bias of primary studies using Cochrane’s RoB 2.0 and ROBINS-I. A random-effects meta-analysis and a meta-regression were performed for continuous and dichotomous outcomes. GRADE approach was used to assess the certainty of the evidence.
Results
Eighteen SRs/MAs, most of them “critically low” and “low” confidence with substantial overlap, included 14 relevant RCTs with a high risk of bias. A cohort study with moderate risk of bias was added. Quantitative synthesis of 595 implants and 281 hemiarches/patients indicates that the use of short implants (< 10 mm) compared to regular implants and BA may reduce implant failure at 1-year follow-up, and marginal bone loss (MBL) at 3-, 5-, and 8-year follow-up; is likely to reduce the risk of biological complications at 1-, 3-, 5-, and 8-year follow-up; and may be the patient's preferred alternative. There is a correlation between bone height, MBL and biological complications.
Conclusions
The available evidence partially suggests that the use of short implants could decrease implant failure, MBL, and biological complications, and increase patient satisfaction. However, given the need for further RCTs and real-world evidence to fully evaluate short- and long-term outcomes, it would be prudent for clinicians to carefully consider the individual needs and circumstances of the patients before deciding whether to use short implants.
Trial registration PROSPERO CRD42022333526
Graphical Abstract
Comments