Beta-defensin index: A functional biomarker for oral cancer detection
Summary
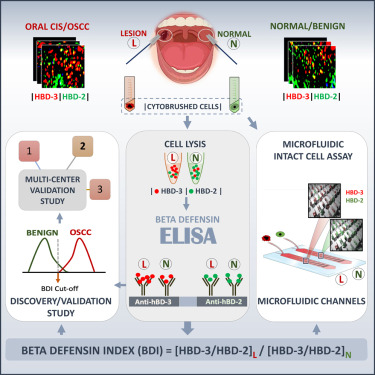
There is an unmet clinical need for a non-invasive and cost-effective test for oral squamous cell carcinoma (OSCC) that informs clinicians when a biopsy is warranted. Human beta-defensin 3 (hBD-3), an epithelial cell-derived anti-microbial peptide, is pro-tumorigenic and overexpressed in early-stage OSCC compared to hBD-2. We validate this expression dichotomy in carcinoma in situ and OSCC lesions using immunofluorescence microscopy and flow cytometry. The proportion of hBD-3/hBD-2 levels in non-invasively collected lesional cells compared to contralateral normal cells, obtained by ELISA, generates the beta-defensin index (BDI). Proof-of-principle and blinded discovery studies demonstrate that BDI discriminates OSCC from benign lesions. A multi-center validation study shows sensitivity and specificity values of 98.2% (95% confidence interval [CI] 90.3–99.9) and 82.6% (95% CI 68.6–92.2), respectively. A proof-of-principle study shows that BDI is adaptable to a point-of-care assay using microfluidics. We propose that BDI may fulfill a major unmet need in low-socioeconomic countries where pathology services are lacking.
Comments